Is Isotretinoin In The Clear After All?
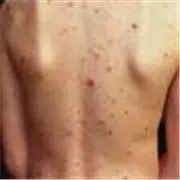
The indicated link between isotretinoin and suicidal thoughts in teenagers might be much better explained by acne severity, propose Norwegian researchers.
Their study of 3775 adolescents revealed that 25.5% of girls and 22.6% of boys 18 or 19 years of age with serious acne thought of suicide, compared with 11.9% of girls and 6.3% of boys with little or no evidence of the condition.
They have suggested that suicidal thoughts and depression which have formerly been linked to acne drugs, specifically isotretinoin, may actually be due to the severity of the condition and its subsequent psychological toll.
Research leader Dr Lars Lien, a psychiatrist with the Institute of Psychiatry, University of Oslo said: “Acne is often identified in late adolescence and is linked with social and psychological issues. Adverse events, including suicidal ideation and depression, which have been associated with treatments for acne might reflect the burden of substantial acne rather than the effects of medication.”
Background:
Isotretinoin, sold by Roche AG as Accutane, is the most potent and effective drug available for treatment of severe acne. One of the severe, treatment-resistant forms that usually responds to isotretinoin is cystic acne. More than 400 Accutane lawsuits claiming the acne medication triggered a varietyof side effects, including inflammatory bowel disease (IBD), Crohn’s disease and ulcerative colitis, have been submitted in a New Jersey mass tort litigation over the last two months. There are now close to 1,600 pending in the New Jersey lawsuit, all of which claim manufacturers Roche AG failed to advise users of the acne drug’s considerable side effects. Nationally, more than 5,000 lawsuits have been filed over the years alleging a number of complications with Accutane (isotretinoin).
Authorized by the Food & Drug Administration (FDA) in 1982, Accutane has been the subject of hot debate for years. It first gained attention in the late 1980’s for causing serious birth defects. It has also been identified as causing psychological problems, and has been linked to hundreds of cases of suicide in the US. Accutane has also been linked with problems of the liver, kidneys, central nervous system, and pancreas, as well as the cardiovascular, musculoskeletal and auto-immune systems.
Roche decided in 2009 to stop producing Accutane, primarily for economic reasons. In announcing the decision, Roche cited the high cost of product liability suits involving the drug as one of the factors in the decision.
The surge in Accutane claims filed in New Jersey arrives on the heels of a court ruling there that found the statute of limitation for such court action should be based on when plaintiffs identified there might be a link between Accutane and their bowel disorder. Roche has lost all seven Accutane cases that have been considered by juries since 2007, including the last three in a row.
LINESPACE
Related blogs:-
- Best Acne Treatment Uk | Acne Home Remedies